 The science of regenerative medicine must embrace engineering, computation and measurement to enable scalable, consistent and cost-effective manufacturing. BioFabUSA, a program of ARMI, is defining the fundamental tenets of good manufacturing processes in the biofabrication industry.
The science of regenerative medicine must embrace engineering, computation and measurement to enable scalable, consistent and cost-effective manufacturing. BioFabUSA, a program of ARMI, is defining the fundamental tenets of good manufacturing processes in the biofabrication industry.
With more than 104,000 children and adults on the national organ transplant waiting list, and an average of 17 people dying each day waiting for an organ transplant, the need to develop regenerative treatments has never been greater.
While those statistics are staggering, they are not news to the Advanced Regenerative Manufacturing Institute (ARMI) and its BioFabUSA program, which is fueling the biomanufacturing economy and advancing the development of life-saving treatments for the world’s most challenging heath conditions. In fact, since it was established in late 2016, BioFabUSA has been working to transform the treatment of traumatic and chronic illness through the scalable, consistent and cost-effective manufacturing of cells, tissues and organs.
ARMI and BioFabUSA integrate innovative cell and tissue cultures with advances in biofabrication, automation, robotics and analytical technologies to create disruptive research and development tools, as well as volume manufacturing processes that are compliant with the United States Food and Drug Administration.
“By combining the power of a large and diverse membership ecosystem with new manufacturing technology, regulatory expertise and state-of-the-art analytical equipment, BioFabUSA is bringing together the best minds and the best tools to advance the field of tissue engineering,” said Maureen Toohey, deputy director of ARMI and BioFabUSA. “We are also building a progressive pipeline of talent through accessible educational and training opportunities, growing the biofabrication workforce at all levels and generating inclusive economic development.”
From Treating to Curing Chronic Conditions
Toohey explained that BioFabUSA’s comprehensive range of capabilities is revolutionizing the field of regenerative medicine, which has had a storied past and promises to improve medical care at the foundation. Fulfilling its potential requires an all-hands-on-deck approach and commitments from leaders in science, medicine, engineering, government, academia and, yes, manufacturing.
 BioFabUSA is a public-private partnership with more than 170 members that represent industry, academia, government and nonprofit organizations.
BioFabUSA is a public-private partnership with more than 170 members that represent industry, academia, government and nonprofit organizations.
According to the U.S. Department of Commerce’s National Institute of Standards and Technology (NIST), regenerative medicine therapy, including cell therapy, gene therapy and therapeutic tissue engineering, provides unprecedented potential to treat, modify, reverse or cure previously intractable diseases, such as cancer and organ failures. According to a NIST report, “This class of therapy has completely changed the paradigm and the trajectory for medical treatment. Broad clinical translation and patient access requires advances in manufacturing technologies and measurements to ensure the safety, quality and consistency of the therapy, and to reduce the cost.”
While the power of regenerative medicine technologies to enhance military readiness, restore injured warfighters and veterans, and improve health outcomes for all Americans has been acknowledged for decades, Toohey noted that even the most promising technologies have fallen short of their full impact. This is due to a variety of factors, including boundaries between traditional scientific disciplines, differences in manual and artisanal research and manufacturing approaches, and fluctuations in funding. This is the set of barriers that BioFabUSA was founded to break down.
 Maureen Toohey, deputy director of ARMI and BioFabUSA
Maureen Toohey, deputy director of ARMI and BioFabUSA
“Realizing the promise of biomanufacturing and regenerative medicine technologies, and delivering therapies at the point of need, requires sustained, capable leadership and the commitment of a coalition with a full compendium of expertise operating in the public interest,” Toohey explained. “Our true value is in pioneering curative therapies for millions across the nation and around the world.”
The delivery of such therapies also requires a trained and prepared national workforce. And BioFabUSA is working to build awareness of this mission at every level, from students to military veterans to industry leaders.
“We are engaging youth-serving community organizations, providing project-based curriculum for middle and high school students, working to launch certificate and registered apprenticeship programming, and engaging higher learning and clinical training institutions to build content that enhances existing curriculum,” Toohey said. “We are working to inform, attract, inspire and recruit all who have a desire to be a part of this industry’s future workforce—part of a transformational future.”
Overcoming Challenges, Chalking Up Wins
There are several key challenges when developing methods of regenerating or engineering human cells, tissues and organs that can restore healthy biological functions.
“The primary challenge facing U.S. regenerative medicine companies in the next two to three years is access to the wraparound support needed to transform discovery science into real-world impact,” asserted Tom Bollenbach, ARMI and BioFabUSA chief technology officer. “Companies need access to advanced manufacturing, engineering, process development and automation expertise, regulatory advisement, and business planning—which is the continuum of capabilities offered by BioFabUSA,” he said.
 Tom Bollenbach, ARMI and BioFabUSA chief technology officer
Tom Bollenbach, ARMI and BioFabUSA chief technology officer
BioFabUSA has implemented multiple automation innovations that have improved the productivity and reliability of cell and tissue manufacturing, including:
A novel liquid-handling automation platform that enables the automated filling of manufactured tissue product into final product containers.
A method to dispense cells tangentially onto the surface of a membrane through microfluidic tubing in a fully sealed bioreactor system, while maintaining final product quality control specifications.
A cryostorage device that securely stores and conducts automated retrieval of samples.
“These automation advancements are a leap forward in biofabrication industry technological capabilities, and the process development team has renewed standard operating procedures to ensure the resultant enhancements in reliability and productivity are shared across projects,” Bollenbach explained. He also said the advancements enhance the scalability, automation and closure of multiple tissue manufacturing processes, purposefully leveraging modularity to maximize productivity. “Specifically, projects that require intermediate or final product containers require delicate manipulation of cells, and/or require cryostorage, are advancing more rapidly due to BioFabUSA’s purposeful application of these automated platforms.”
The Tissue Foundry
To demonstrate the power of a scalable, modular, automated and closed manufacturing (SMAC) system, BioFabUSA created the Tissue Foundry, a methodology that involves analyzing manual processes, designating automation modules for unit operations, creating custom-engineered components, integrating the modules into an automated and closed process, and confirming that the process delivers critical quality attributes.
 Lisa Larkin, STEL Technologies co-founder and CEO
Lisa Larkin, STEL Technologies co-founder and CEO
“BioFabUSA has closed and automated multiple manual, time-intensive tissue engineering processes,” Bollenbach said. “Among these are Epibone’s autologous bone tissue manufacturing process, which uses isolated and expanded patient-derived mesenchymal stem cells (MSCs) and decellularized bovine bone to provide anatomically tailored bone reconstruction for patients with trauma, bone-related genetic defects or reconstruction after the treatment of cancer and other illnesses.”
BioFabUSA conducts deep process work with its members and continually applies relevant ecosystem capabilities to transition manual processes to SMAC manufacturing. “When a commercial off-the-shelf solution does not exist for a specified process need, BioFabUSA convenes members for focused technical innovation,” Bollenbach said. “Closure and automation of the seeding, feeding and harvesting of MSCs during expansion in the Epibone process has transformed a previously artisanal process into a scalable manufacturing approach—ultimately enabling the production of affordable future therapies.”
ARMI member STEL Technologies LLC, Ann Arbor, Mich., develops bioengineered anterior cruciate ligaments (ACLs), which are tissues in the knee that serve as a stabilizing force and are often torn or overstretched. STEL (Skeletal Tissue Engineering Laboratory) Technologies was founded by researchers at the University of Michigan to help revolutionize the treatment of soft tissue injuries. Working with ARMI, STEL is on the path toward scaling out and mass producing tissue-engineered regenerative medicine (TERM) products for clinical use, according to co-founder and CEO Lisa Larkin.
“My tissue-engineered ACL product and the bioreactor designed to fabricate the product were the first two technologies selected by ARMI and BioFabUSA to develop a Tissue Foundry around,” Larkin explained. The Tissue Foundry line is displayed annually at the Meeting in the Millyard, a three-day event (June 6-8, Manchester, N.H.) featuring expert-led panels and more than 40 presentations by pioneers in the field.
From Vision to Validation
“Dean sold me on the vision in the early days,” said Michael Golway, CEO of Louisville, Ky.-based Advanced Solutions Life Sciences LLC. “It was a wonderful win-win scenario in which some of the best in the world at different aspects of biomanufacturing would create this community of SMEs, coming together to showcase innovation, improve innovation and participate in new innovation necessary to realize the long-term vision Dean had.”
 Michael Golway, CEO of Advanced Solutions Life Sciences LLC
Michael Golway, CEO of Advanced Solutions Life Sciences LLC
Golway is referring to ARMI founder Dean Kamen, an engineer, inventor and entrepreneur who holds more than 1,000 patents—including ones for the first insulin pump for diabetics and a stair-climbing wheelchair—and started the For Inspiration and Recognition of Science and Technology (FIRST) robotics community to inspire the next generation of great inventors. But Kamen is perhaps best known for developing the two-wheeled, self-balancing Segway personal transporter.
“Back in 2017, we were the first company to join the consortium started by ARMI,” said Golway, whose company is focused on the discovery, design and development of integrated hardware, software and biological solutions for the biomedical and biotech fields of science and industry. “Since I met Dean, I’ve been amazed at his ability to connect the dots at a large level. By and large, that vision he shared years ago is coming true,” he added.
In fact, Advanced Solutions was so inspired by that vision that a team located a research laboratory on the campus of the ARMI office in Manchester, N.H., and just renewed the lease for another three years.
“I’m excited about realizing innovation that will be applied for human use in a way that brings profound and positive change. We’re right on the cusp of that,” Golway said, adding that he sees tremendous value in ARMI and BioFabUSA as a platform to amplify the work of its members.
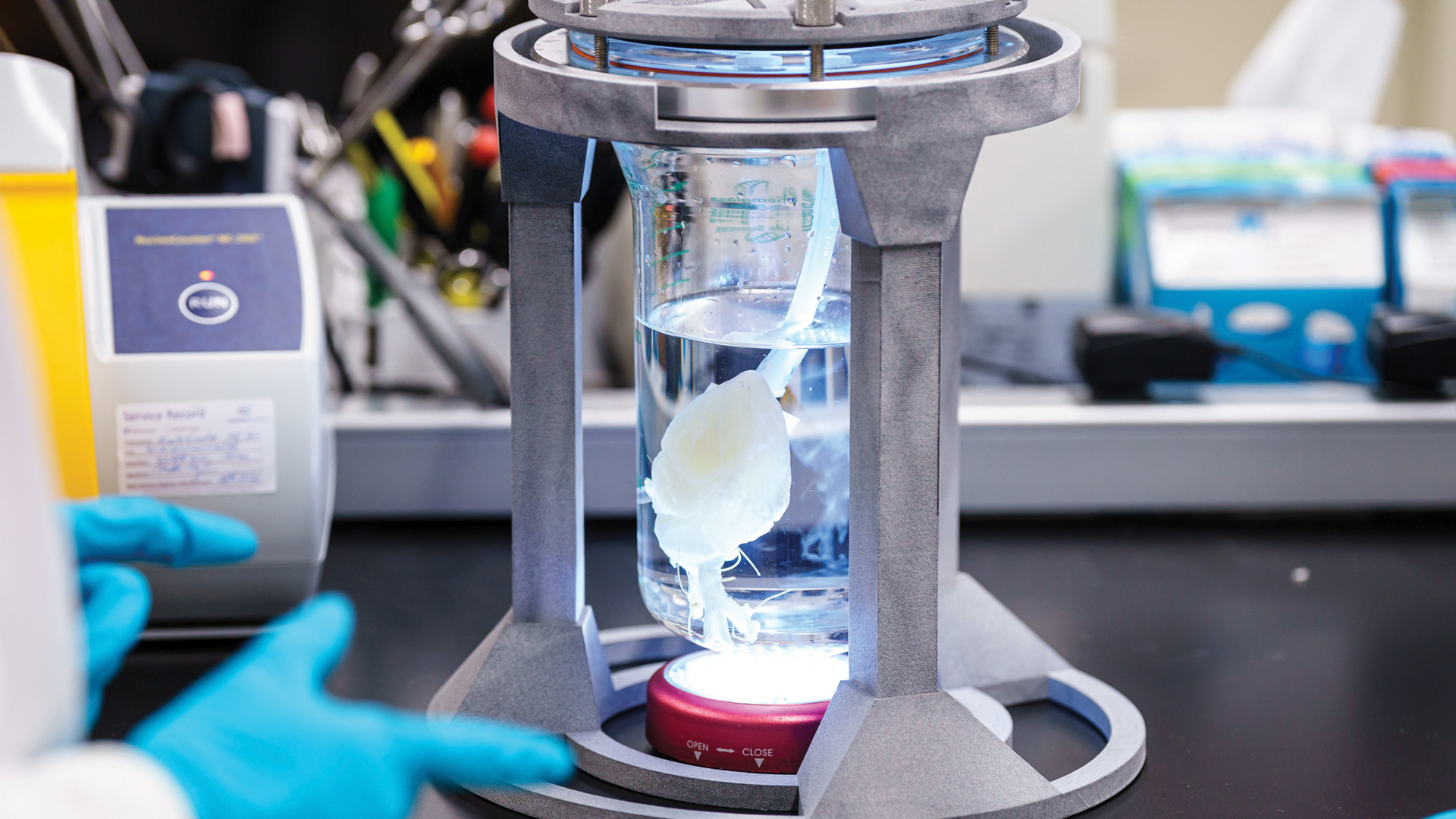
A stellar example of the program: Doris Taylor, PhD, CEO and founder of Organomet Bio, used Advanced Solutions’ BioAssemblyBot (BAB) to create the world’s first bioengineered human heart.
 Dean Kamen is the founder of ARMI and a prolific engineer, inventor and entrepreneur.
Dean Kamen is the founder of ARMI and a prolific engineer, inventor and entrepreneur.
Dubbed the most advanced robotic 3D biology platform, BAB 3D prints human cells and has a six-axis robot arm that creates structures, holds tools and performs assembly. Taylor taught BAB a method of injecting 350 billion cells into a heart scaffold that facilitated the automation and creation of a human heart fast enough to enable on-demand, bio-engineered transplants, which has the potential to make the traditional transplant waiting-list process a thing of the past.
Leveling the Playing Field
When it comes to the commercialization of products, Larkin views BioFabUSA as a significant collective enterprise in moving the needle. “They have convened expertise in all areas needed to accomplish this endeavor, including funding process development of promising tissue-engineered technologies or technologies necessary for the success of a Tissue Foundry line, FDA regulatory advice for companies with TERM products, deep tissue characterization of products to obtain data for interactions with the FDA, collection and testing of non-invasive monitoring of tissues for subsequent product release criteria, and annual meetings to foster collaborations with other ARMI members.”
 Doris Taylor, PhD, CEO and founder of Organomet Bio used BioAssemblyBot (BAB) to create the world’s first bioengineered human heart.
Doris Taylor, PhD, CEO and founder of Organomet Bio used BioAssemblyBot (BAB) to create the world’s first bioengineered human heart.
One of the main challenges is closed system fabrication at high volume. “The highest cost for TERM product fabrication is the payment of expert technicians,” Larkin said. “With the automation of the products on the Tissue Foundry line, the need for highly paid experts will be significantly reduced, creating opportunities for non-degree technicians.”
Larkin expects the BioFab Startup Lab will continue the trend of game-changing capabilities ARMI has “to create a sustainable industry for biofabrication.”
The goal of the BioFab Startup Lab, launched through a grant from the U.S. Economic Development Administration, is to bring promising technologies to market. The lab provides focused, tailored business planning and access to the capabilities offered by BioFabUSA. “The Startup Lab is guiding companies for this new industry from incubation through market readiness,” Toohey noted.
“It’s difficult to go from ideation to something that benefits humans,” Golway added. “If something is going to go into a human, the process through the FDA to make that happen is challenging. The reality is a lot of smart people are going through the same processing steps to create something usable in the clinical world. BioFab is a mechanism that will allow companies to shortcut that process. They don’t have to reinvent the wheel and that levels the playing field for small and mid-sized manufacturers.”
Larkin agrees. “BioFab is a very collegial environment that allows for exchange of ideas between its members. This is uncommon in the world of small businesses where everyone is worried about keeping trade secrets and are scared to share their technologies with others.”
It is this force multiplier effect and potential impact that makes the promise of ARMI and BioFabUSA, as well as its robust membership community, so exciting to explore.
The new 25,000-sq-ft Startup Lab facility that supports process development, co-located with BioFabUSA in the Manchester Millyard, simplifies the transition of products from early stage development to commercial manufacturing. “With the proposed organ delivery network and Vertiport also in the Millyard at the Armory, an organ can be manufactured and on its way to save a life in under an hour,” said Bollenbach. “This facility will deliver on the promise of regenerative manufacturing, providing therapy developers a pathway from early to late-stage clinical trials and commercial launch.”
By creating the path for curative therapies to come to market, there’s no limit to how many lives can be saved, and the quality of life improved, in the months and years ahead.